Reuters - Merck Co Incs cancer drug Keytruda failed a late-stage trials main goals of slowing disease progression and. Pembrolizumab Keytruda and nivolumab Opdivo are drugs that target PD-1 which can help boost the immune response against cancer cells.
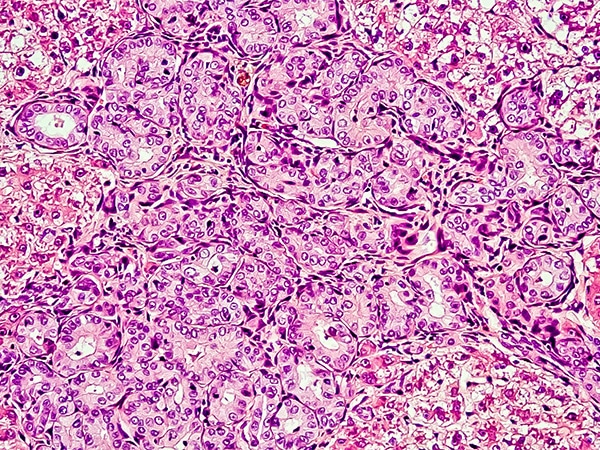 Expanding Immunotherapy Options For Liver Cancer Patients The Aacr
Expanding Immunotherapy Options For Liver Cancer Patients The Aacr
However its efficacy and security in the treatment of advanced liver cancer is still under investigation.
Keytruda liver cancer. The success rate of Keytruda depends upon the type of cancer you have the stage of your cancer your previous treatments. Mercks Keytruda flunks liver cancer confirmatory trial 3 months after accelerated approval The company said it had shared data from the Phase III study with the FDA. A study of Keytruda pembrolizumab in patients with a difficult-to-treat type of liver cancer suggests that the treatment may extend the time before their disease progresses.
Head and neck cancer. Keytruda pembrolizumab is an inhibitor of programmed cell death receptor-1 PD-1 which was approved to treat advanced melanoma and nonsmall cell lung cancer patients who do not respond to other treatment. Keytruda is used alone or in combination with other medicines to treat certain types of cancer such as.
Skin cancer melanoma or Merkel cell carcinoma. These drugs can be used in people with advanced liver cancer who have previously been treated such as with the targeted drug sorafenib Nexavar. This can shrink some tumors or slow their growth.
Merck CoMSD has won approval in the US for PD-1 inhibitor Keytruda as a treatment for hepatocellular carcinoma HCC the most common form of liver cancer. Listing a study does not mean it has been evaluated by the US. In its first-quarter financial filing Merck said the application for Keytruda and Lenvima was already under review at the FDABut before the target review completion date the FDA approved a combination therapy from Roche.
Primary mediastinal large B. Its a rare setback for Mercks market-leading cancer therapy Keytruda which brought in sales of 33 billion in the first three months of this year alone. The Keytruda development program is also progressing well and the drug is being studied for more than 30 types of cancer in more than 900 studies including more than 500 combination studies.
According to the results of a study presented at the recent gastrointestinal cancer symposia Keytruda pembrolizumab is an active treatment for advanced liver cancer in patients who have already been treated with Nexavar sorafenib 1 and has not been approved by. Data presented at the 2018 American Society of Clinical Oncology Gastrointestinal Cancers Symposium on Jan. And Canada is an immune checkpoint inhibitor that works to prevent a mechanism used by cancer cells to evade immune responses.
Advertentie Unlimited access to Liver Cancer market reports on 180 countries. Merck Co Incs cancer drug Keytruda failed a late-stage trials main goals of slowing disease progression and extending the life of patients with a common type of liver cancer. Keytruda developed by Merck known as MSD outside the US.
In general PD-1 immune checkpoint inhibitors like Keytruda have been shown to significantly prolong overall survival OS in some patients over a wide range of cancer types. Advertentie Unlimited access to Liver Cancer market reports on 180 countries. 19 showed that Keytruda stabilized the disease in a significant percentage of patients.
Merck Eisais Keytruda-Lenvima combo stonewalled in liver cancer after Roches first-in-class green light The FDA has slapped a complete response. The applications are seeking approval of KeytrudaLenvima combination for the first-line treatment of unresectable hepatocellular carcinoma HCC the most common type of liver cancer. But its important to know that these cancer treatments do not work for everyone.
Tap into millions of market reports with one search. Tap into millions of market reports with one search. It specifically targets the PD-1 receptor at the surface of immune T-cells and prevents it from binding to the PD-L1 protein on cancer cells removing the brakes from immune cells.
February 19 2019 211 PM. Pembrolizumab Keytruda in Advanced Hepatocellular Carcinoma The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
 Keytruda Sbla Granted Priority Review For Advanced Hepatocellular Carcinoma Infectious Disease Advisor
Keytruda Sbla Granted Priority Review For Advanced Hepatocellular Carcinoma Infectious Disease Advisor
 Keytruda Leads To Durable Response For Some People With Advanced Liver Cancer Cancer Health
Keytruda Leads To Durable Response For Some People With Advanced Liver Cancer Cancer Health
 Merck S Keytruda Flunks Liver Cancer Confirmatory Trial 3 Months After Accelerated Approval Medcity News
Merck S Keytruda Flunks Liver Cancer Confirmatory Trial 3 Months After Accelerated Approval Medcity News
 Liver Cancer Progression Slowed By Keytruda In Phase 2 Trial
Liver Cancer Progression Slowed By Keytruda In Phase 2 Trial
 Keytruda Immunotherapy For Treatment Of Liver Cancer Cancerconnect
Keytruda Immunotherapy For Treatment Of Liver Cancer Cancerconnect
 Immunotherapies Liver Cancer Treating Effects Confirmed In Real World Studies Pharma 기사본문 Kbr
Immunotherapies Liver Cancer Treating Effects Confirmed In Real World Studies Pharma 기사본문 Kbr
 Fda Approves Keytruda For Liver Cancer Cancer Health
Fda Approves Keytruda For Liver Cancer Cancer Health
 Fda Declines To Approve Merck And Eisai S Liver Cancer Combo Therapy Pharmalive
Fda Declines To Approve Merck And Eisai S Liver Cancer Combo Therapy Pharmalive
 Bristol Myers Opdivo Loses Fda Panel S Favor In Liver Cancer But Squeezes Out Merck S Keytruda In Stomach Cancer Fiercepharma
Bristol Myers Opdivo Loses Fda Panel S Favor In Liver Cancer But Squeezes Out Merck S Keytruda In Stomach Cancer Fiercepharma
 Merck S Keytruda Hits The Skids In Pivotal Trial Putting Its Liver Cancer Nod At Risk Fiercepharma
Merck S Keytruda Hits The Skids In Pivotal Trial Putting Its Liver Cancer Nod At Risk Fiercepharma
 Keytruda Approved For Liver Cancer Patients Who Had Used Nexavar
Keytruda Approved For Liver Cancer Patients Who Had Used Nexavar
 Keytruda Opdivo S Retention Of Hepatocellular Carcinoma Indication May Depend On Ongoing Studies Pink Sheet
Keytruda Opdivo S Retention Of Hepatocellular Carcinoma Indication May Depend On Ongoing Studies Pink Sheet
 Merck S Keytruda Fails Liver Cancer Trial Indivior To Get Suboxone Competition S P Global Market Intelligence
Merck S Keytruda Fails Liver Cancer Trial Indivior To Get Suboxone Competition S P Global Market Intelligence
 Advanced Hepatocellular Carcinoma In Patients Previously Treated With Sorafenib Patients Keytruda Pembrolizumab
Advanced Hepatocellular Carcinoma In Patients Previously Treated With Sorafenib Patients Keytruda Pembrolizumab

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.