414 male 684 had CD and 316 had UC. The second review presented in Barcelona was a US-specific analysis assessing the real-world use of immunosuppressives IM across a total of 567 patients which also provide information on the safety profile of ENTYVIO.
 Entyvio Reviews Vedolizumab A New Medication For Ibd
Entyvio Reviews Vedolizumab A New Medication For Ibd
Vedolizumab Entyvio is a humanized monoclonal antibody α4β7 integrin-receptor antagonist indicated for the treatment of adult patients with moderately to severely active ulcerative colitis or Crohns disease.
Entyvio reviews from patients. These findings are consistent with clinical trial data and support the long-term benefit-risk profile of vedolizumab. On 20 March 2014 the EU Committee for Medicinal Products for Human Use CHMP issued a positive opinion recommending the granting of a marketing authorisation in Europe. For severe Crohns.
Also before I went on Entyvio I asked a lot of questions about it and in my opinion it seemed like the safe choice. All these medicines can be scary due to bad side effects. Patient preference favoring treatment discontinuation was improved during treatment with Entyvio or Stelara compared with anti-TNF therapy in patients.
See what others have said about EntyvioIntravenous including the effectiveness ease of. And maintenance of clinical response and remission by Entyvio vedolizumab in pediatric patients 6 to 17 years of age with moderately to severely active Crohns disease who have failed conventional therapy. Intestinal and extraintestinal symptom relief and remission were identified by patients as being most important when considering Crohns disease treatment.
Of the 567 patients 586 female. Nine percent of patients reported serious adverse events. This article reviews the pharmacological properties of intravenous infusions of vedolizumab and its clinical efficacy in adult patients with these diseases.
The second review presented in Barcelona was a US-specific analysis assessing the real-world use of immunosuppressives IM across a total of. Show ratings reviews for. 43 of those users who reviewed Entyvio reported a positive effect while 34 reported a negative effect.
I think she is on a good medicine and time will tell if it will help. EntyvioIntravenous received an overall rating of 10 out of 10 stars from 2 reviews. Started Stelara as second choice when request for Entyvio was denied by insurance.
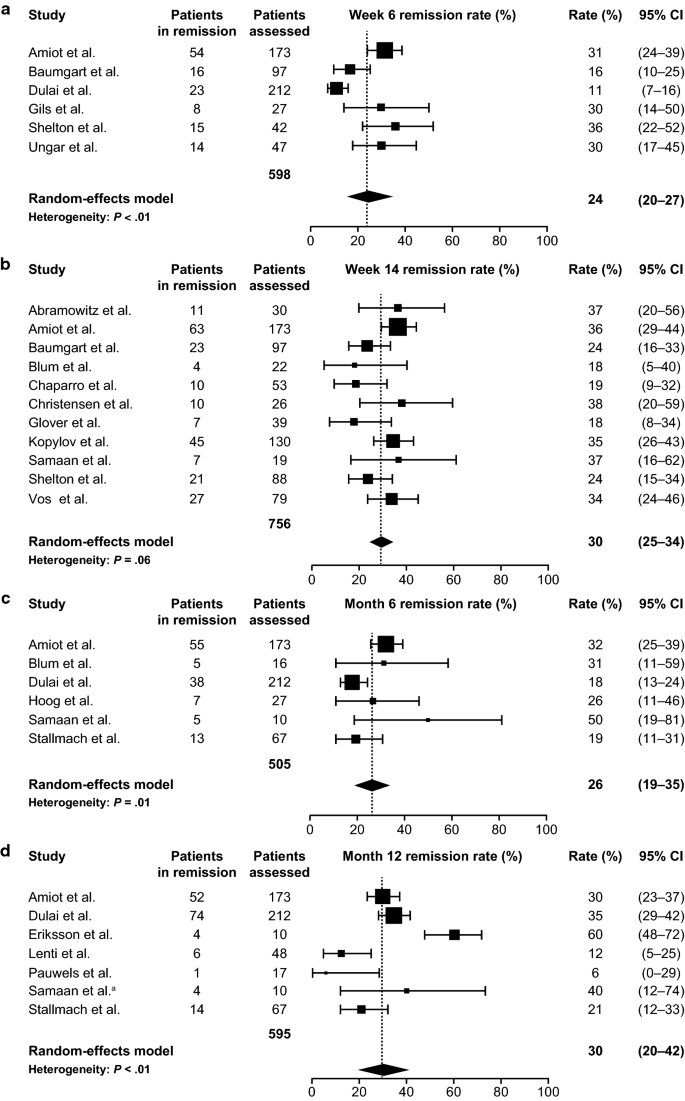
In phase III clinical trials patients with. The side effects that I have with it are unusual so there is a good. Vedolizumab demonstrated real-world effectiveness in patients with moderate-to-severely active UC or CD with approximately one-half and one-third of patients respectively in remission at treatment month 12.
Conduct a randomized placebo-controlled blinded multicenter study of the induction. I know it is tough but she will feel better and it could very well be Entyvio that does it for her. Patients felt that more biologic options would be beneficial despite remarkable results observed with current biologics.
At the time the TGA considered this application a similar application was under review in the European Union EU the USA Canada and Switzerland. Vedolizumab is available in single-use vials containing 300 mg of vedolizumab. User Reviews for Entyvio to treat Ulcerative Colitis Entyvio has an average rating of 57 out of 10 from a total of 54 ratings for the treatment of Ulcerative Colitis.
Thought I would have to. It is administered via IV infusion and must be reconstituted and diluted prior to. The mucosal healing systematic review analysed 26 studies that reported mucosal healing andor endoscopic improvement rates in patients with moderate to severe CD taking Entyvio.
Went from liquid bowel movements to formed no blood no puss no urgency. I am very happy and shocked with results so far using Stelara. May 2026 Final Report Submission.
Seven days after first infusion noticed symptoms beginning to improve. The most common definition of mucosal healing was the absence of all ulcers andor erosions. 41 of those users who reviewed Entyvio reported a positive effect while 35 reported a negative effect.
Twenty-six percent of CD patients at six months and 37 percent at 12 months achieved mucosal healing with Entyvio. Ten days after first infusion ALL SYMPTOMS ARE GONE. All Conditions 52 reviews Crohns Disease 29 reviews Ulcerated Colon 11 reviews Ulcerative Colitis currently Without Symptoms 10 reviews.
In a direct comparison with Humira Entyvio won out as the top choice for second line therapy for patients with ulcerative colitis who failed therapy with Remicade according to. Vedolizumab has been previously reviewed through the CADTH Common Drug Review CDR for the treatment of ulcerative colitis. The mean age at index was 44 and on average patients initiated ENTYVIO.
August 2020 Study Completion. CADTH COMMON DRUG REVIEW Clinical Review Report for Vedolizumab Entyvio 8 The objective of this review is to perform a systematic review of the beneficial and harmful effects of vedolizumab SC injection for the treatment of adult patients with moderately to severely active UC who have had an inadequate response to loss of response to or were.
