These courses will give experienced TMF professionals in roles such as Clinical Project Manager TMF Lead TMF Project Manager or Head of Trial Master File a more in-depth understanding of complex TMF concepts and responsibilities while also ensuring a broad understanding of how to apply their knowledge to unique TMF challenges and lifecycles. Works with sponsors to provide compliant electronic trial master files eTMFs What is a Trial Master File TMF Comprised of essential documents that allow for the evaluation of the conduct of a trial and the quality of data produced.
 Our Top 10 Q A On Trial Master File Eccrt
Our Top 10 Q A On Trial Master File Eccrt
This course will ensure participants comply with the latest regulations 31A-1-3 for Trial Master Files TMF.

Trial master file online training. Other ancillary materials can be. These live instructor-led sessions are designed to be highly interactive and can be attended by individual attendees or groups at one low cost. This is a professionally developed interactive and engaging online course for anyone involved in running a clinical trial or working within a clinical trial environment.
This course is based on the international E6 ICH Good Clinical Practice regulations and is a complete training solution for all individuals that need to acquire GCP knowledge. The HSRAA Trial Master File TMF Essentials training course is suitable for anyone with responsibility for establishing or maintaining trial master files whether in paper or electronic format. Attendees will gain an understanding of how to comply with the regulatory requirements that are pertinent to the maintenance of the TMF and how to.
The Trial Master File is a collection of the essential documents for a sponsor to record how they have fulfilled their obligations for a clinical trial. Our free GCP training can also serve as a refresher course. SCOPE Applicable to all clinical research projects undertaken at Western Health WH including.
A Barnett Interactive Web Seminar offers you a seamless secure multimedia learning experience. Dedicated to trial master file. Our webinar platform combines the convenience of online training with the live interactivity of the classroom.
A final close-out of a trial can only be done when the monitor has reviewed both investigatorinstitution and sponsor files and confirmed that all necessary documents are in the appropriate files. The course covers a broad array of topics specific to TMF from the regulatory requirements for the TMF ALCOA as it applies to TMF TMF responsibilities TMF structure inspection preparedness regulatory findings and a wide range of practical issues associated with management of the TMF. It demonstrates good clinical practice GCP compliance and validates that the organizations clinical practices are in alignment with other widely accepted regulatory requirements.
Enclosed are zip files comprising the documents for the GCP Training days 1 and 2 as well as example ICFs Binder cover pages tools etc. The Trial Master File should contain all the Essential Documents required by ICH-GCP that are appropriate for your clinical trial. 30 Dated May 2019 Page 2 of 33 1.
This globally recognized trial master file management plan allows organizations to effectively compile the minimum list of essential data and documentation for regulatory purposes. Procedures in case of an involved party closing down its business for any reason. The monitors responsibility is to monitor the conduct of a research trial.
The clinical trial master file paper andor electronic Draft adopted by GCP Inspectors Working Group GCP IWG 30 January 2017. THE TRIAL MASTER FILE AND ESSENTIAL DOCUMENTS Version. Set Up and Maintenance.
Quizzes case studies break-out sessions and assessments are often incorporated into the training. The activities of set-up maintenance and monitoring will be discussed using check-lists and case studies to highlight common deficiencies and potential solutions. Such as central training documents and central e-mail repository.
With MasterTrack Certificates portions of Masters programs have been split into online modules so you can earn a high quality university-issued career credential at a breakthrough price in a flexible interactive format. AIM To describe the procedures related to the maintenance of the Study Site Master FileTrial Master File TMF and associated essential documents. Trial master files should be established at the beginning of the trial both at the investigatorinstitutions site and at the sponsors office.
What is a Trial Master File TMF SpecialistThis is one of our Clinical Research positions explained videos where we break down a role in clinical research. Understand the purpose and extent of the Trial Master File and how to best manage it. This module provides focused training on areas such as feasibility assessment site selection pre-study visit site initiation subject recruitment and retention trial master file TMF and clinical trials budgeting.
The Trial Master File. Trial Master File TMF for Sponsors. No travel no travel expenses and no time away from the office.
This Trial Master File training will review the essential elements of a TMF for clinical trials. The Code of Federal Regulations states in 21 CFR 31250 that Sponsors are responsible for ensuring that the investigation s is conducted in accordance with the general investigational plan and protocols contained in the IND. This clinical compliance training will review what issues one needs to consider if ones organization is thinking about introducing an IT solution for the management of trial master file content.
What is a trial master file in clinical researchhttpwwwTheClinicalTrialsguru. Study Monitoring Close Out. It is suitable for anyone carrying out or involved in clinical research and clinical trials.
 It S Ten O Clock Do You Know Where Your Trial Master File Is Life Science Training Institute
It S Ten O Clock Do You Know Where Your Trial Master File Is Life Science Training Institute
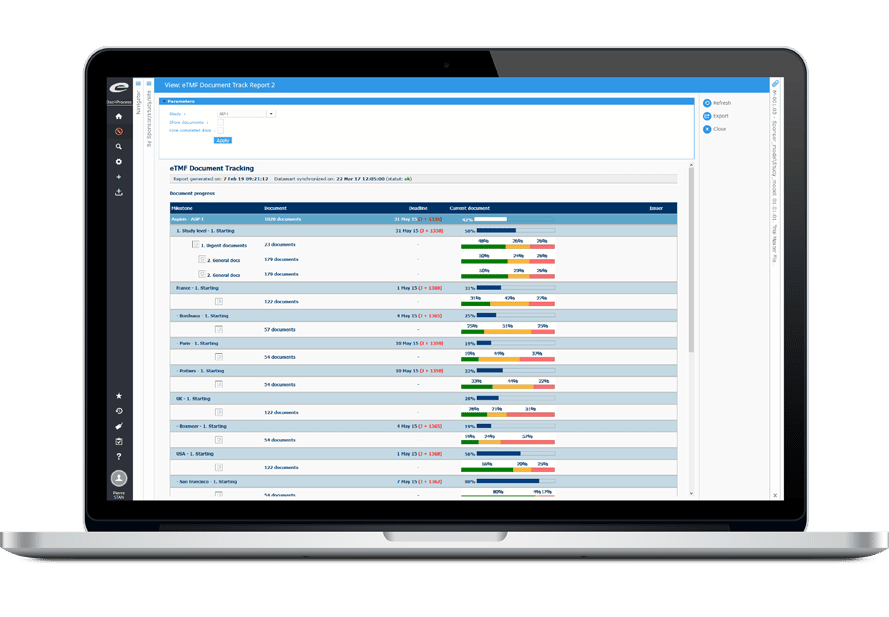 Etmf Electronic Trial Master File Software Ennov Software For Life
Etmf Electronic Trial Master File Software Ennov Software For Life

Electronic Trial Master File Etmf Specification Version 1 0
 Essential Documents Online Training Course Training Online 4u
Essential Documents Online Training Course Training Online 4u
 5 Pillars Of The Trial Master File Tmf Lmk Clinical Research Llc
5 Pillars Of The Trial Master File Tmf Lmk Clinical Research Llc
Trial Master File Tmf Translation Services Stepes
 Electronic Trial Master File Etmf
Electronic Trial Master File Etmf
Https Tmfrefmodel Files Wordpress Com 2018 03 Tmf Rm Deliverable User Guide V1 2018 03 16 Pdf
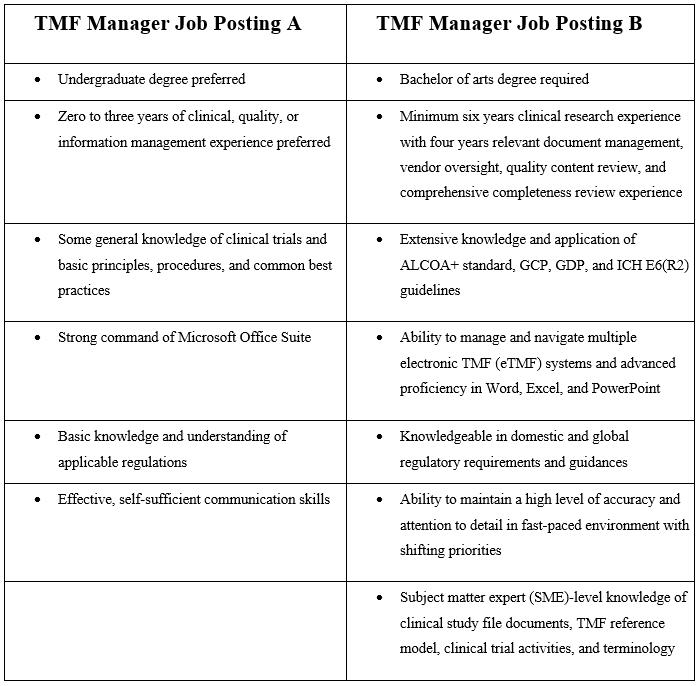 What Skills Are Needed To Effectively Manage An Etmf
What Skills Are Needed To Effectively Manage An Etmf
![]() E Tract Secure Collaboration In Clinical Trials
E Tract Secure Collaboration In Clinical Trials
 Tmf University Educational Courses Trial Master File Become A Tmf Specialist
Tmf University Educational Courses Trial Master File Become A Tmf Specialist
 Ensuring A Quality Trial Master File
Ensuring A Quality Trial Master File
 Clinical Trials Hurdles Session 4 Trial Master Files 2015 Sc Ctsi
Clinical Trials Hurdles Session 4 Trial Master Files 2015 Sc Ctsi

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.