JUDE MEDICAL and the nine-squares symbol are trademarks and service marks of St. Jude Childrens Research Hospital produces both types of devices.
 Complex Remote Monitoring With St Jude Medical Merlin Net Download Scientific Diagram
Complex Remote Monitoring With St Jude Medical Merlin Net Download Scientific Diagram
Jude Medical MR Conditional system includes a St.
St jude device. Jude Medical or one of its subsidiaries. The AF Suppression algorithm is a feature on St. For information on programming refer to.
These documents may be revised periodically. Abbott formally known as St. Jude Medical pulse generators listed in the table below.
Jude Medical MR Conditional device connected to one or more St. 7Fr x 110cm 7500. Jude Medical Recalls Assurity and Endurity Pacemakers for Potential Moisture Ingress Causing Electrical Short and Reduced Battery Life Medical Device.
Jude Medical Technical Services at 800-722-3774. To evaluate a fully resorbable device to treat blocked arteries below the knees or critical limb ischemia CLI in people battling advanced stages of peripheral artery disease PAD. The transmitter supports the St.
Jude Medical Product Manuals Access online digital product and treatment information for patients or healthcares professionals to view download or print. For a list of the devicelead combinations that have been tested refer to the St. Jude Medical MR Conditional device connected to one or more St.
Jude Medical devices that is clinically proven to reduce ATAF burden and improve quality of life by pacing in the atrium just above the. If you have any questions on this topic please contact St. Placed over the device to prevent inappropriate therapy.
Jude Medical MR Conditional leads. Device Description This manual describes the St. Jude Medical which is not affiliated with St.
Jude Medical Inquiry AFocus II Diagnostic Catheter - 7Fr x 110cm. These devices can be programmed with Merlin Patient Care System equipped with Model 3330 version 2122 or greater software. Once the treatment session is completed the magnet should be removed.
Jude Medical today announced CE Mark approval of its next-generation quadripolar device the Quadra Assura MP cardiac resynchronization therapy defibrillator CRT-D. Jude Medical implantable cardiac devices pacemakers defibrillators and resynchronization devices provide pacing for slow heart rhythms and electrical shock or pacing to stop. The Apex locator is battery operated and should not interfere with pacemakers or ICDs.
Federal USA law restricts this device to sale by or on the order of a physician. For a list of the devicelead combinations that have been tested see the devicelead combinations. Unless otherwise noted indicates that the name is a trademark of or licensed to St.
Jude Medical Current R RF and Promote R RF family of devices and works in conjunction with the St. This is the first Investigational Device Exemption IDE trial in the US. Implantable Devices A device such as an implantable defibrillator or pacemaker can be used to suppress the symptoms of AF with low-dose electrical energy.
Jude Medical MR Conditional system includes a St. The device features MultiPoint Pacing MPP technology that enables physicians to pace multiple locations on the left side of the heart. Jude Medical LLC and its related companies.
A problem with the lithium batteries used in some of their ICDs and CRT-Ds led the company to recall close to 400000 of the devices in 2016 after two patients died. Jude Medical Pacel Flow Directed Pacing Catheter Rt Heart Curve-5Fr x 110cm-Non-Expired. 5Fr x 110cm 5000.
Jude Medical MR Conditional leads.
St Jude Medical Launches Spinal Cord Stimulation System In Europe Neuronews International
 Fda Harshly Criticizes Abbott St Jude For Failure To Address Ep Device Safety Daic
Fda Harshly Criticizes Abbott St Jude For Failure To Address Ep Device Safety Daic
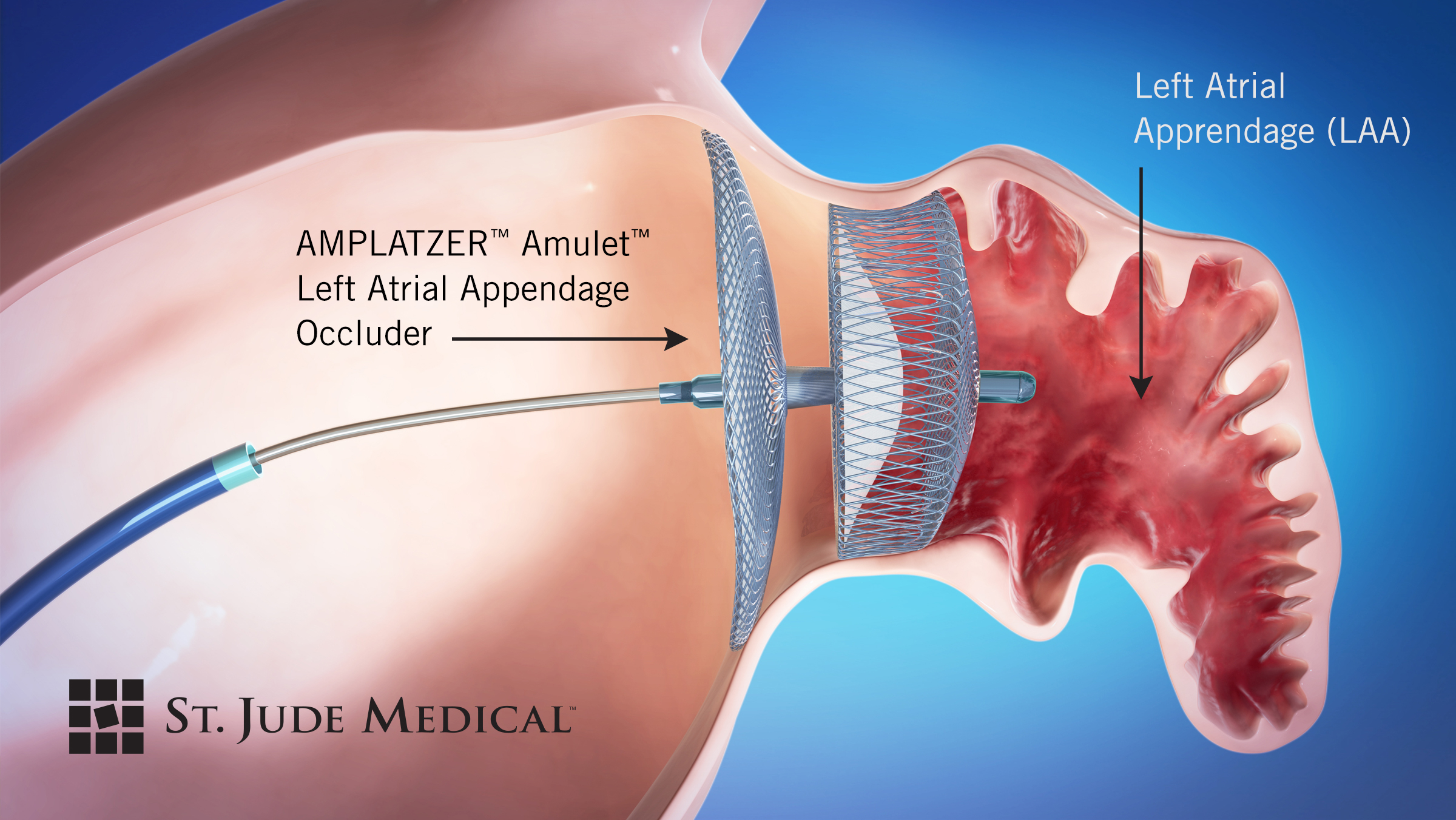 Grosste Beobachtungsstudie Des St Jude Medical Amplatzer Amulet Left Atrial Appendage Occluder Bis Dato Zeigt Grossen Implantationserfolg Und Ein Solides Sicherheitsprofil Business Wire
Grosste Beobachtungsstudie Des St Jude Medical Amplatzer Amulet Left Atrial Appendage Occluder Bis Dato Zeigt Grossen Implantationserfolg Und Ein Solides Sicherheitsprofil Business Wire
St Jude Medical The World Of Implantable Devices
 St Jude Medical Alleged Merlin Home Crash Is Actually A Security Feature Massdevice
St Jude Medical Alleged Merlin Home Crash Is Actually A Security Feature Massdevice
 Top Global Medical Device Companies Medical Product Outsourcing
Top Global Medical Device Companies Medical Product Outsourcing
 St Jude Medical Erhalt Weltweit Erste Ce Zulassung Fur Systeme Zur Tiefen Hirnstimulation Bei Primarer Und Sekundarer Dystonie Business Wire
St Jude Medical Erhalt Weltweit Erste Ce Zulassung Fur Systeme Zur Tiefen Hirnstimulation Bei Primarer Und Sekundarer Dystonie Business Wire
 Trading In Stock Of Medical Device Paused After Hackers Team With Short Seller Ars Technica
Trading In Stock Of Medical Device Paused After Hackers Team With Short Seller Ars Technica
 St Jude Medical Design Innovation Consultancy
St Jude Medical Design Innovation Consultancy
 St Jude Medical Played Down Defibrillator Failures For Years F D A Says The New York Times
St Jude Medical Played Down Defibrillator Failures For Years F D A Says The New York Times
 St Jude Medical Refutes Muddy Waters Device Security Allegations Medical Product Outsourcing
St Jude Medical Refutes Muddy Waters Device Security Allegations Medical Product Outsourcing
 Fda To Patients With St Jude Pacemakers Update Needed To Keep Hackers Out Of Devices Healthcare It News
Fda To Patients With St Jude Pacemakers Update Needed To Keep Hackers Out Of Devices Healthcare It News
 St Jude Medical Defibrillators Devices At Risk Recalls
St Jude Medical Defibrillators Devices At Risk Recalls
 Market Report Calls Into Question St Jude Medical Ep Device Safety Cybersecurity Daic
Market Report Calls Into Question St Jude Medical Ep Device Safety Cybersecurity Daic

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.